Blue Cross Blue Shield of North Dakota is committed to providing members with access to high-quality healthcare consistent with evidence-based, nationally recognized clinical criteria and guidelines. To ensure value to our members, we're changing the way we manage certain clinician administered drugs that process under the medical benefit. The Medical Pharmacy Solutions team at Prime Therapeutics Management will administer the new process.
How It Works
Providers and their staff will have the opportunity to obtain prior authorization to help streamline medical drug administration and service. NOTE: This new process will not change the retail pharmacy prior authorization process through the pharmacy benefit.
For determination of services requiring prior authorization follow the steps below:
- Search for In Network Prior Authorization ›
- Fill out required fields and submit
- Follow the instructions listed under the additional procedure information section
- Shown below is a service requiring a medical pharmacy prior authorization
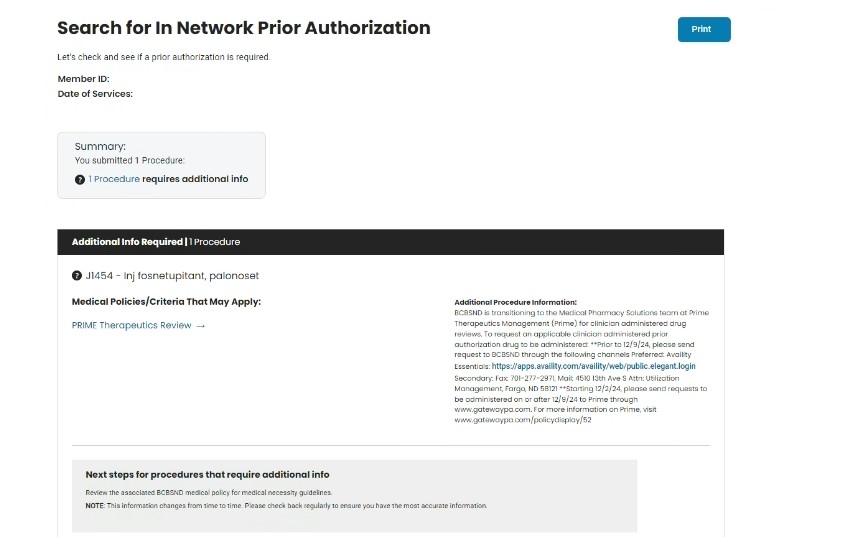
4. Access https://www.gatewaypa.com/
5. For additional policy information reference https://www.gatewaypa.com/policydisplay/52
FOR PRIME PRIOR AUTHORIZATIONS
- Providers and their staff will have the opportunity to obtain prior authorization to help streamline medical drug administration and service online at www.GatewayPA.com or by phone at 800-424-1708.
- If a prior authorization request does not initially have sufficient evidence to be approved, it is pended to be clinically reviewed by PrimeMPS clinical pharmacists.
- If the initial clinical reviewer finds the request meets clinical criteria, the initial clinical reviewer can approve the prior authorization request.
- If the initial clinical reviewer cannot find sufficient evidence to approve the request, the clinical reviewer will schedule a peer-to-peer conversation between the provider and Prime peer clinical reviewer, who is a board- certified physician. The Prime peer clinical reviewer will render the final determination based on the information received. Note: Prime initial clinical reviewers are clinical pharmacists.
FOR POST-SERVICE CLAIM EDIT REVIEWS
- There will be medical drugs that will be reviewed using post-service claim edit (PSCE) policies.
- For certain drugs billed under the medical benefit, post-service/pre-payment claims edits may be applied based on the medication, diagnosis, Maximum billable units allowed (as specified in the policy for that drug).
- For a complete list of the post-service/pre-payment claims and policies, please visit www.gatewaypa.com/policydisplay/52.
Medical Pharmacy Policy Updates and Requirements
Stay informed about new precertification requirements, preferred product categories, and post-service claim policies to ensure seamless claims processing and compliance. Due to the constantly changing medical pharmacy category, please refer to www.gatewaypa.com/policydisplay/52 for the most up-to-date information.
Education on Demand video presentation
Education on Demand video presentations
faq