On Dec. 9, 2024, we went live with our new Medical Pharmacy prior authorization process with the Medical Pharmacy Solutions team at Prime Therapeutics Management (Prime). Providers are now required to use the Prime GatewayPA when submitting medical pharmacy drugs requiring prior authorization for Commercial members.
With the new process change, we are seeing an increase of prior authorizations being sent that do not require prior approval. Refer to our Medical Pharmacy Policy Updates and Requirements section of our provider website for an update on which products no longer require prior authorization.
The following drugs no longer require prior authorization and will be reviewed post service using post service claim edit policies: Aralast NP, Asparlas, Beleodaq, Besponsa, Botox, Brineura, Daxxify, Dysport, Empliciti, Flolan, Fulphila, Glassia, Myobloc, Neulasta, Prolastin-C, Remodulin, Supprelin LA, Triptodur, Veletri, Xeomin, Xiaflex and Zemaira.
*Note: This list may be subject to change, providers should reference https://www.gatewaypa.com/policydisplay/52 for additional information.
For determination of medical pharmacy drugs requiring prior authorization or post service claim review follow the steps below:
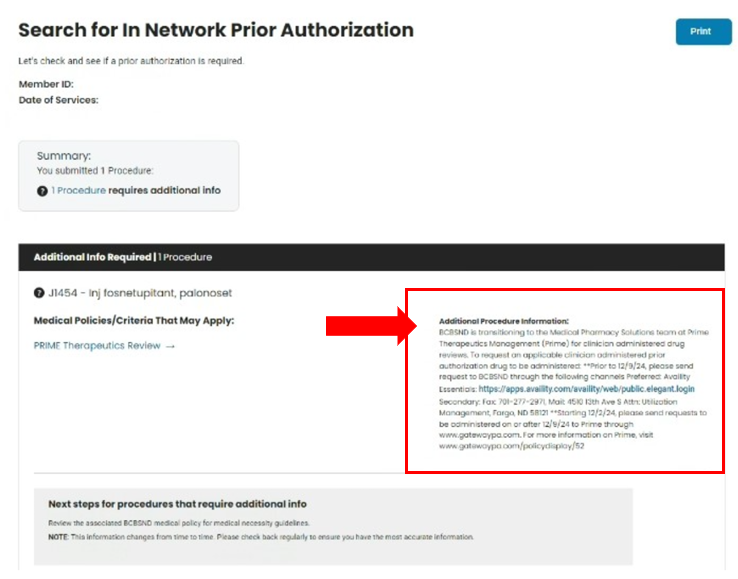
- Search In Network Prior Authorization (Provider) | BCBSND
- Fill out required fields and submit
- National Provider ID
- Member
- Date of service
- Procedure Code or Service Description
- Submit
- If the service requires authorization or post-service claim review, follow the instructions listed under the Additional Procedure information section
- Shown below is a medical pharmacy drug requiring prior authorization or post service claim review.