Impacting our Commercial line of business only.
Blue Cross Blue Shield of North Dakota (BCBSND) is informing our provider community that the U.S. Food and Drug Administration (FDA) has approved multiple biosimilar products for the reference brand name medication, Humira. Biosimilar products for Humira are indicated to reduce signs and symptoms of various autoimmune disorders. These biosimilar agents are available in the market and offer additional safe and cost-effective options for management of autoimmune disorders.
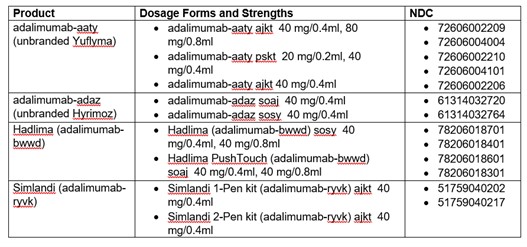
Effective Jan. 1, 2025, adalimumab-aaty (unbranded Yuflyma), adalimumab-adaz (unbranded Hyrimoz), Hadlima, and Simlandi will be the preferred biosimilar products on all BCBSND formularies. Humira will be removed from formularies and will be a non-preferred medication. Current coverage for Humira will remain the same through Dec. 31, 2024.
Prior authorization (PA) approvals for Humira will be terminated Dec. 31, 2024, and new prior authorization approvals for adalimumab-aaty (unbranded Yuflyma), adalimumab-adaz (unbranded Hyrimoz), Hadlima, and Simlandi will be entered in its place effective Jan. 1, 2025, through the original Humira PA expiration date.
Utilization Management policies will be updated to reflect the formulary changes and will be available through www.bcbsnd.com on Jan. 1, 2025.
Preferred Products Effective Jan. 1, 2025: